Abstract
Background: Outcome of AML pts is known to be impacted by genetic factors such as cytogenetic abnormalities and gene mutations and sociodemographic factors such as age and race. Neighborhood factors are increasingly recognized as important drivers of disparities in cancer outcomes. Neighborhood socioeconomic deprivation has been associated with worse survival of numerous cancers, but studies in AML are lacking. Understanding the role of social deprivation in AML outcomes, and its impact in the context of known prognostic factors could identify areas that may benefit from additional health care resources.
Methods: We analyzed the clinical and molecular features of 1,242 younger AML (age range, 17-59 y) pts similarly treated with intensive cytarabine/daunorubicin-based chemotherapy on frontline Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology protocols between 2001 and 2013. A neighborhood social deprivation index (SDI) was assigned to all pts based on pt reported zip code of residence. SDI was population weighted with resulting scores (1-100) corresponding to US zip code percentiles of SDI (e.g., 100 = top 1% of US population in zip codes of highest deprivation). We defined 2 SDI levels, low (1-50 n=664) and high (51-100, n=578), that were analyzed for associations with clinical and molecular features and outcomes.
Results: Demographic and baseline clinical features did not differ between high and low SDI pts, except for a higher percentage of self-reported non-Hispanic Black pts residing in high compared to low SDI areas (15% v 2%, p<.001). In contrast, a higher percentage of self-reported non-Hispanic White AML pts resided in low compared to high SDI areas (94% v 79%). A lower percentage of pts residing in high SDI areas underwent allogeneic transplant in 1 st complete remission (CR) compared to those in low SDI areas (10% v 17%, p<.001).
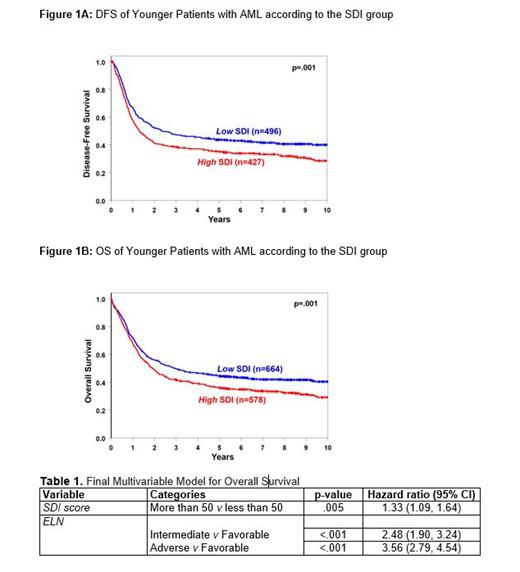
Concerning clinical outcomes, there were no significant differences in early death (7% v 6%) or CR (67% for both) rates between low and high SDI pts. However, pts residing in high SDI areas had shorter disease-free survival (DFS; median: 1.3 v 2.4 y, p=.001; Fig. 1A) and overall survival (OS; median: 1.9 v 2.9 y, p<.001; Fig. 1B). Notably, White AML pts residing in high SDI areas had worse DFS (3-y rates: 37% v 46%, p=.003) and OS (3-y rates: 42% v 48%, p=.006) than those low SDI areas. In contrast, there were no associations between SDI and survival among Black AML pts, indicating that systemic racism may outweigh poverty-associated survival impact.
We next assessed whether differences in molecular features between high and low SDI groups may help explain the observed survival disparities (n=656 pts). While we found no statistically significant differences in the frequencies of known prognosis-associated gene mutations by SDI levels, there was a trend towards lower frequencies in genes belonging to the cohesion complex and methylation-associated genes (both p=.06), indicating possible differences in underlying disease biology.
Percentages of high and low SDI pts did not differ significantly within any of the 3 genetic-risk groups in the 2017 European Leukemia Net (ELN) classification. However, when we analyzed outcomes of pts within the ELN groups, we found that among pts belonging to the ELN Favorable-risk group, high SDI score was associated with shorter OS (p=.03) than low SDI score, but this was not true for ELN Intermediate- or Adverse-risk pts. Lastly, in multivariable analysis for OS in younger AML pts, a high SDI score associated with shorter OS (HR: 1.28, CI: 1.10-1.48, p=.01; Table 1), after correction for ELN groups (p<.001), thereby highlighting that a pts socioeconomic status predicts outcome independently from our routinely used molecular-based, clinical risk stratification.
Conclusion: In younger AML pts, residence in areas with high socioeconomic deprivation was associated with poor survival, especially for pts classified in the ELN favorable-risk group. With the exception of patient racial-ethnic identity and receipt of allogeneic transplant in first CR, SDI did not associate with clinical or molecular characteristics. Thus, area social deprivation among younger AML pts should be further investigated to overcome potentially avoidable survival disparities.
Support: U10CA180821, U10CA180882 U24CA196171; Clinicaltrials.gov Identifiers: NCT00048958, NCT00899223, NCT00900224; https://acknowledgments.alliancefound.org
Mims: Leukemia and Lymphoma Society's Beat AML clinical study: Consultancy, Research Funding; Aptevo: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Glycomemetics: Research Funding; Kartos Pharmaceuticals: Research Funding; Xencor: Research Funding; Genentech: Consultancy; Abbvie: Consultancy; BMS: Consultancy; Kura Oncology: Consultancy; Syndax Pharmaceuticals: Consultancy; BMS: Consultancy; Jazz Pharmaceuticals: Consultancy; Aptevo: Research Funding. Blachly: KITE: Consultancy, Honoraria; INNATE: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Blum: Nkarta: Research Funding; Celyad Oncology: Research Funding; Abbvie: Honoraria; AmerisourceBergen: Honoraria; Xencor: Research Funding; Forma Therapeutics: Research Funding; Leukemia and Lymphoma Society: Research Funding; Syndax: Honoraria. Uy: Agios: Consultancy; AbbVie: Consultancy; Novartis: Consultancy; GlaxoSmithKline: Consultancy; Genentech: Consultancy; Macrogenics: Research Funding; Astellas: Honoraria, Speakers Bureau; Jazz: Consultancy. Stone: Abbvie: Consultancy; Actinium: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Aprea: Consultancy; Arog: Consultancy, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Boston Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; Foghorn Therapeutics: Consultancy; Gemoab: Membership on an entity's Board of Directors or advisory committees; Glaxo Smith Kline: Consultancy; Innate: Consultancy; Jannsen: Consultancy; Jazz: Consultancy; Macrogenics: Consultancy; Novartis: Consultancy, Research Funding; Onconova: Consultancy; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy. Byrd: Newave: Membership on an entity's Board of Directors or advisory committees; Vincerx Pharmaceuticals: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, Syndax: Consultancy, Honoraria. Paskett: Pfizer: Research Funding; Merck: Research Funding. Eisfeld: Karyopharm (spouse): Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal